Regency View:
Update
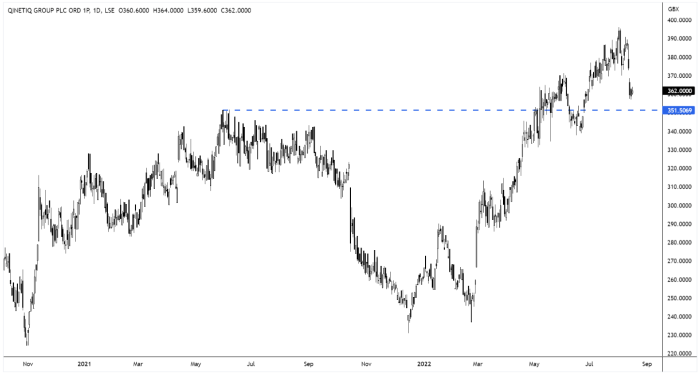
Qinetiq wins US contract and announces US acquisition
It’s been a good couple of weeks for Qinetiq’s (QQ.) US growth prospects…
Earlier this month, Qinetiq announced it has entered into an agreement to acquire Avantus Federal LLC from NewSpring Holdings, for an enterprise value of $590m (£483m).
Avantus is a leading provider of mission-focused cyber, data analytics and software development solutions to the US Department of Defense, Intelligence Community, Department of Homeland Security and other Federal civilian agencies.

In the 12 months to 30 June 2022, Avantus generated revenues of $298m, adjusted earnings (EBITDA) of $35.5m and adjusted operating profit of $32m. The deal will generate a tax benefit for Qinetiq of approximately $70m.
The US acquisition has been followed by a US contract win…
Qinetiq announced last week that it had won a contract to provide technical services to the US Army.
The five-year contract, worth up to $45m, will provide services for the Development Command (DEVCOM) Command, Control, Computers, Communications, Cyber, Intelligence, Surveillance and Reconnaissance (C4ISR) at the Fort Belvoir Prototyping Integration Facility (PIF).
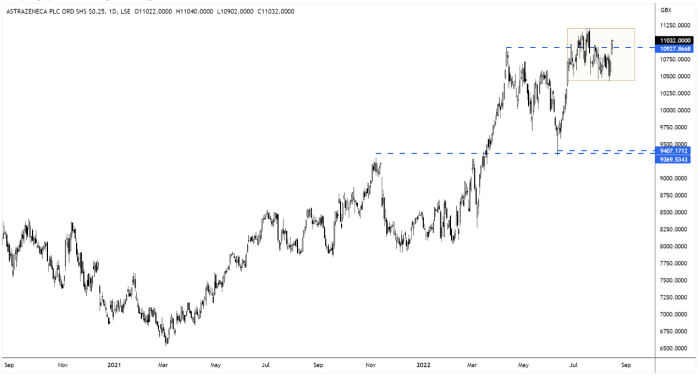
AstraZeneca says data confirms Enhertu benefit in breast cancer patients
Enhertu, AstratZeneca (AZN) and Daiichi Sankyo’s breast cancer drug continues to gather positive momentum…
On Monday, AstraZeneca said a late-stage trial had confirmed the benefit of Enhertu in patients with an advanced form of the disease who had been previously treated with another therapy.
In a 600-patient trial called DESTINY-Breast02, Enhertu was compared against a treatment pre-determined by physicians in people with HER2-positive metastatic breast cancer.

The study showed that Enhertu met the main goal of statistically significant and clinically meaningful improvement in progression-free survival, a measure of how long a person can live without their disease worsening. The drug also improved overall survival, a key secondary goal.
This positive study news comes on the back of Enhertu securing faster-than-expected US approval, in patients with a particular form of breast cancer.
The new US Food and Drug Administration (FDA) approval – for patients with ‘HER2-low’ breast cancer – has opened a large, new multibillion-dollar market for AstratZeneca and Daiichi Sankyo.
Disclaimer:
All content is provided for general information only and should not be construed as any form of advice or personal recommendation. The provision of this content is not regulated by the Financial Conduct Authority.